BACKGROUND: In newly diagnosed AML, high remission rates are typically achieved with IC, but the response is often transient, and detectable residual disease in the bone marrow post-chemotherapy is predictive of early relapse. Emerging data show that the identification of ≥ 0.1% MRD by multiparameter flow cytometry (MFC) in patients with AML in remission after IC is an important prognostic marker that may help guide treatment (Tx) decisions. CC-486 is an oral hypomethylating agent that allows for extended dosing schedules to prolong drug exposure over the Tx cycle. In the QUAZAR AML-001 Maintenance Trial, Tx with CC-486 300 mg QD for 14 days/28-day Tx cycle was associated with significantly improved overall (OS) and relapse-free survival (RFS) vs. placebo (PBO) in patients (pts) with AML in first remission after induction chemotherapy ± consolidation. Samples for MFC were obtained prior to randomization and serially throughout the study to assess the impact of MRD on OS and RFS, and to evaluate rates of conversion from MRD positivity (+) to negativity (-) in the CC-486 and PBO arms.
METHODS: Eligible pts aged ≥ 55 years with AML were randomized 1:1 to CC-486 300 mg or PBO within 4 months of achieving first complete remission (CR) or CR with incomplete blood count recovery (CRi). MFC assessments of bone marrow aspirates were performed centrally at screening; at cycles 3, 6, 9, 12, 15, 18, 21, 24, 30, and 36; and as clinically indicated. Samples were analyzed with a panel of 22 cell surface markers using an MRD+ cutoff of ≥ 0.1% (per ELN MRD guidelines). For pts MRD+ at baseline (BL; ie, at randomization), an MRD response was defined as achievement of MRD- for ≥ 2 consecutive assessments. MRD- duration was calculated from the time of randomization (for pts MRD- at BL) or from the first of ≥ 2 consecutive MRD- tests (for pts MRD+ at BL), until the last MRD- assessment (for pts who became MRD+) or Tx discontinuation. OS, RFS, and MRD- durations were estimated using Kaplan-Meier methods. Multivariate (MV) Cox regression analyses were performed to evaluate the association of BL MRD status (MRD+ vs. MRD-) and randomized Tx arm (CC-486 vs. PBO) with OS and RFS.
RESULTS: The MRD-evaluable cohort comprised 463/472 randomized pts (98.1%; CC-486, n=236; PBO, n=227) who had samples available for evaluation at BL and at ≥ 1 post-BL visit. At BL, 43% of pts (n=103) in the CC-486 arm and 50% (n=116) in the PBO arm were MRD+. Overall, BL characteristics were similar between MRD+ and MRD- pts: median ages were 69 (range 55-84) and 68 (55-86) years, respectively; 84% and 88% had intermediate-risk cytogenetics at diagnosis; 52% and 46% of pts had an ECOG PS of 0; and 79% and 82% received ≥ 1 cycle of consolidation after induction.
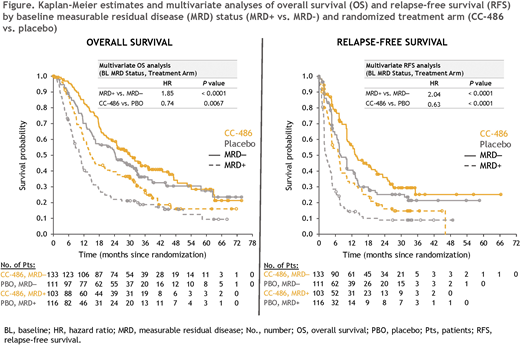
CC-486 Tx resulted in improved OS from time of randomization compared with PBO in pts who were either MRD+ (median 14.6 vs. 10.4 mo, respectively; HR 0.69 [95%CI 0.51, 0.93]) or MRD- (median 30.1 vs. 24.3 mo; HR 0.81 [0.59, 1.12]) at BL. Median RFS was also extended with CC-486 vs. PBO for both MRD+ (7.1 vs. 2.7 mo, respectively; HR 0.58 [95%CI 0.43, 0.78]) and MRD- pts (13.4 vs. 7.8 mo; HR 0.71 [0.52, 0.98]). In MV analyses, BL MRD status (MRD+ vs. MRD-) was significantly associated with OS (HR 1.85; P < 0.0001) and RFS (HR 2.04; P < 0.0001), and CC-486 showed a significant Tx benefit vs. PBO on both OS (HR 0.74; P = 0.0067) and RFS (HR 0.63; P < 0.0001) independent of MRD status at BL (Figure).
The median duration of MRD negativity was extended with CC-486 vs. PBO: 11.0 vs. 5.0 mo, respectively (HR 0.62 [95%CI 0.48, 0.78]). Tx with CC-486 also resulted in a higher rate of MRD response (MRD+ to MRD-) vs. PBO: 37% vs. 19%, respectively. Among MRD responders, 9/38 patients (24%) in the CC-486 arm achieved MRD negativity > 6 mo after randomization, compared with only 1/22 patients (5%) in the PBO arm.
CONCLUSIONS: The QUAZAR AML-001 Maintenance Trial was the first prospective, randomized trial to include long-term longitudinal assessment of MRD in older patients with AML in remission. In both treatment arms, MRD+ status (≥ 0.1%) after induction ± consolidation was associated with significantly shorter OS and RFS compared with MRD- status. Approximately one-fourth of MRD responders treated with CC-486 achieved MRD negativity > 6 mo after study entry, suggesting that CC-486 could induce MRD negativity after prolonged MRD+ status. Maintenance Tx with CC-486 substantially improved OS and RFS independent of MRD status at BL.
Roboz:Otsuka: Consultancy; AstraZeneca: Consultancy; Orsenix: Consultancy; Astellas: Consultancy; Argenx: Consultancy; Actinium: Consultancy; Sandoz: Consultancy; Roche/Genentech: Consultancy; Jazz: Consultancy; Eisai: Consultancy; Celltrion: Consultancy; MEI Pharma: Consultancy; Helsinn: Consultancy; Epizyme: Consultancy; Jasper Therapeutics: Consultancy; Cellectis: Research Funding; Trovagene: Consultancy; Takeda: Consultancy; Astex: Consultancy; Amphivena: Consultancy; Agios: Consultancy; Abbvie: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Bayer: Consultancy; Array BioPharma: Consultancy; Daiichi Sankyo: Consultancy. Ravandi:Abbvie: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Macrogenics: Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding. Wei:Pfizer: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria; Walter and Eliza Hall Institute of Medical Research: Patents & Royalties: AW is eligible for royalty payments related to venetoclax; Roche: Honoraria; Amgen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau; Abbvie: Honoraria, Research Funding, Speakers Bureau; Servier: Consultancy, Honoraria, Research Funding; Macrogenics: Honoraria; Astra Zeneca: Honoraria, Research Funding. Dombret:Menarini: Consultancy; Janssen: Consultancy; Cellectis: Consultancy; Shire-Baxalta: Consultancy; Immunogen: Consultancy; Otsuka: Consultancy; Abbvie: Consultancy; Astellas: Consultancy; Daiichi Sankyo: Consultancy; Servier: Consultancy, Research Funding; Sunesis: Consultancy; Amgen: Consultancy, Research Funding; Jazz Pharma: Consultancy, Research Funding; Celgene: Consultancy; Nova: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Döhner:Astex: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; AROG: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; GEMoaB: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Oxford Biomedicals: Consultancy, Honoraria; Sunesis: Research Funding; Pfizer: Research Funding; Roche: Consultancy, Honoraria; Jazz: Consultancy, Honoraria, Research Funding; Helsinn: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Thol:Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Voso:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Porkka:Novartis: Consultancy, Honoraria, Research Funding; BMS/Celgene: Honoraria, Research Funding. La Torre:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Skikne:Bristol Myers Squibb: Current Employment. Kumar:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Dong:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Beach:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Risueño:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Named in BMS (before Celgene) patent filings related to predictive patient response biomarkers in hematological malignancies. Lopes de Menezes:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Ossenkoppele:Novartis: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Roche: Consultancy; J&J: Consultancy, Research Funding; Agios: Consultancy; Jazz: Consultancy; Astellas: Consultancy; Daiichi Sayko: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal